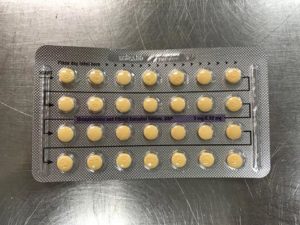
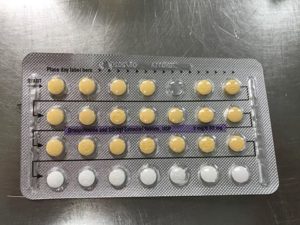
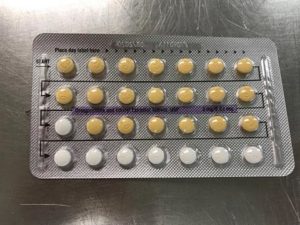
Apotex Corporation has issued a voluntary recall of Drospirenone and Ethinyl Estradiol 3MG/0.03MG tablets.
This pill is sometimes used as a generic substitute for the brands Yasmin, Ocella, Syeda, Yaela, Zarah. The recall is being issued as blister packs may have incorrect tablet arrangements and/or an empty blister pocket.
The following lot numbers are affected:
| Lot Number | Expiration Date | Strength | Configuration |
| 7DY008A | 8/2020 | 3mg/0.03mg | Contains 21 yellow |
| 7DY009A | 8/2020 | 3mg/0.03mg | active tablets and 7 |
| 7DY0101A | 8/2020 | 3mg/0.03mg | white placebo |
| 7DY011A | 8/2020 | 3mg/0.03mg | tablets |
Due to the packaging error, a patient may miss a dose or take a placebo instead of an active tablet. This may cause a patient to not get the desired results from the tablets.
We are actively working to identify possibly affected patients. In the meantime, if you have NOT started the pill pack, contact your pharmacy for a replacement pack. If you have started an affected pill pack, please contact Virginia Women’s Center 804.288.4084 for further instructions
Pictures of the affected packs are shown below.